Adapter dimers are generated by self-ligation of sequencing adapters that are preferentially amplified due to their small size. Adapter dimer contamination appears as a peak at ~150 bp (below), and is caused by low input DNA, inefficient adapter ligation, and/or using excess beads during library purification. Adapter dimers should represent no more than 5% of the total 200-700 bp fragment yield as determined on a Bioanalyzer/Tapestation trace (i.e. % Integrated in trace is less than 5%).
To minimize adapter dimers, keep adapter ligation reagents on ice during ligation setup. If adapter dimers are >5% of your library, they should be removed using the bead:DNA ratios outlined in the Application Notes for our CUTANA™ DNA Purification Beads (EpiCypher 21-1407)
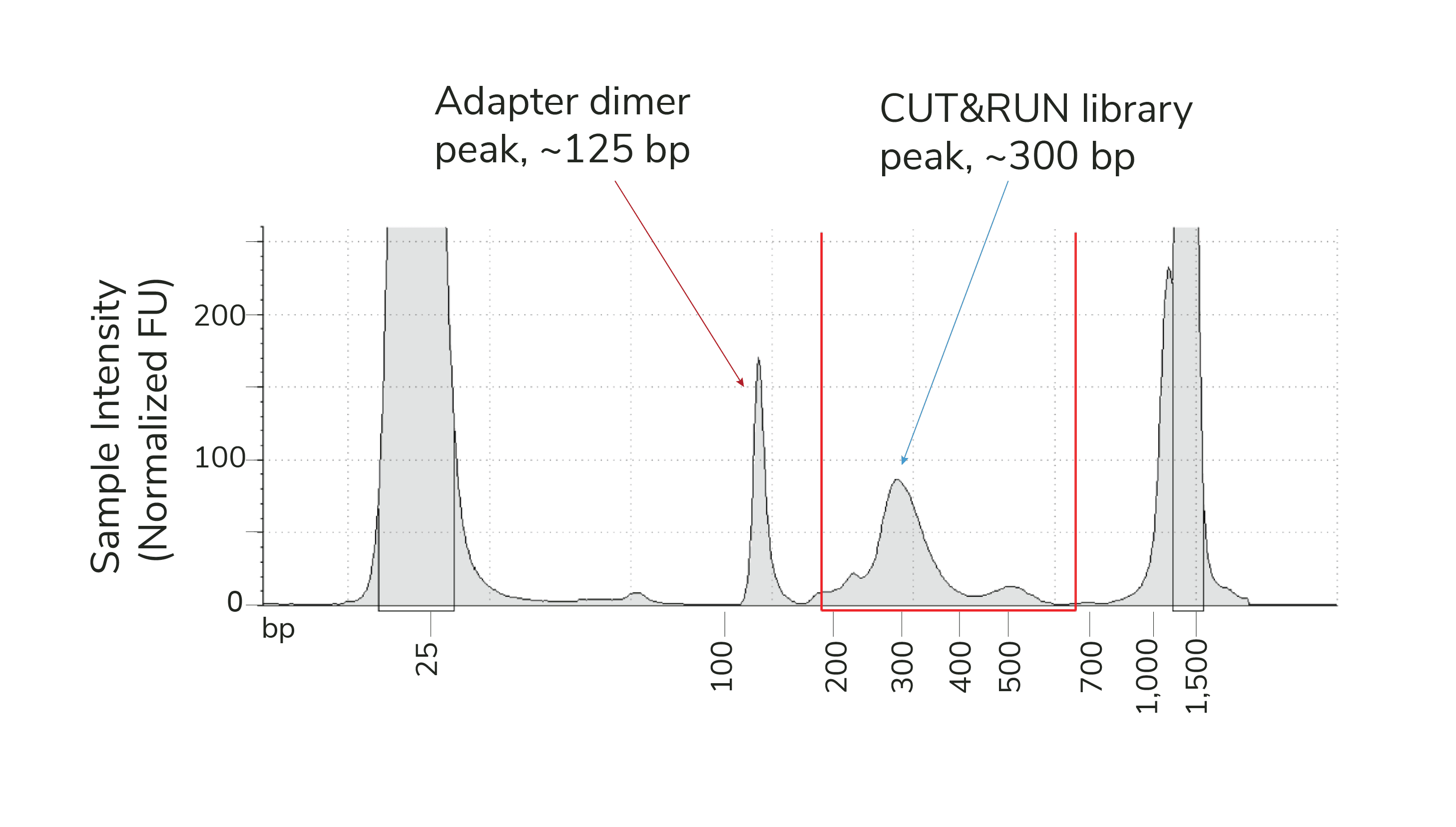
Figure 1. Example TapeStation trace from CUT&RUN H3K27me3 library containing an adapter dimer peak (~150 bp, red arrow) and expected library peak (~300 bp, blue arrow). Red lines denote the 200-700 bp range, used to determine library concentration.