Important Notes
Nuclei Extraction Buffer is prepared fresh on the day of nuclei harvest.
The CUTANA™ Nuclei Extraction Protocol is optimized for CUT&RUN and CUT&Tag workflows. For CUT&RUN, start with 500,000 cells per reaction; for CUT&Tag, start with 100,000 cells per reaction. Harvest 10% excess cells to account for sample loss.
Reconstitute Protease Inhibitor Tablets with molecular biology-grade water to make a 25X stock. If using EpiCypher Protease Inhibitor Tablets, follow reconstitution instructions on product page or technical data sheet.
The Pre-Nuclei Extraction Buffer and prepared Nuclei Extraction Buffer are very viscous; pay careful attention to preparation notes for both to ensure that they are adequately mixed.
Materials Needed
Reagent | Source |
|---|---|
Pre-Nuclei Extraction Buffer | |
1M Spermidine | |
Protease Inhibitor Tablets, EDTA-free | |
Phosphate Buffered Saline (PBS) | Any vendor |
0.4% Trypan Blue | Invitrogen T10282 |
Brightfield or phase microscope + hemacytometer slides | Any vendor |
Nuclei Prep Protocol
Prepare Nuclei Extraction Buffer in a fresh tube. Per reaction, combine 235 µL Pre-Nuclei Extraction Buffer, 0.13 µL Spermidine, and 9.8 µL 25X Protease Inhibitor.
If preparing buffer for fewer than 8 reactions, dilute the 1M Spermidine stock 1:10 in molecular biology-grade water and add 1.3 μL per reaction.
Note: The Pre-Nuclei Extraction Buffer is very viscous. Mix well prior to combining (ideally using a pipette, as vortexing can cause bubbles).
Place final Nuclei Extraction Buffer on ice.
Count fresh or frozen cells and confirm starting cell integrity, morphology, and viability by Trypan Blue staining. It is important that cells have good starting viability. For K562 cells, we aim for >90% viability.
Harvest cells. For CUT&RUN, harvest 500,000 cells per reaction; for CUT&Tag, harvest 100,000 cells per reaction. Harvest 10% excess cells to account for sample loss.
Spin cells at 600 x g for 3 min at room temperature (RT). Remove supernatant and resuspend cells in 100 µL per reaction cold Nuclei Extraction Buffer. Pipette mix the Nuclei Extraction Buffer thoroughly before resuspending.
Incubate on ice for 10 min.
Spin at 600 x g for 5 min at 4ºC. Remove and discard supernatant. The pellet should change in appearance from sticky, pale yellow (cells) to white and fluffy (nuclei).
PLEASE NOTE: This step varies depending on which assay you are performing:
In CUT&RUN: Gently resuspend nuclei in 105 µL per reaction cold Wash Buffer.
In CUT&Tag: Gently resuspend nuclei in 105 µL per reaction cold Wash Buffer 1.
If cryopreserving (for either assay): Gently resuspend nuclei in 105 uL per reaction cold Nuclei Extraction Buffer and skip to Nuclei Preservation and Thawing Protocol below.
Take a 10 µL aliquot to examine nuclear integrity by Trypan Blue staining. Isolated nuclei will stain Trypan Blue positive and should have clear borders and minimal debris. Intact cells will be bright white and round (see Figure 1). For accurate nuclei counts, record "dead" cell numbers on an automated cell counter or manually count blue stained nuclei.
Proceed to desired assay (CUT&RUN or CUT&Tag)—nuclei in Wash Buffer/Wash Buffer 1 can be directly added to activated ConA beads. If freezing nuclei for later use, see cryopreservation protocol below.
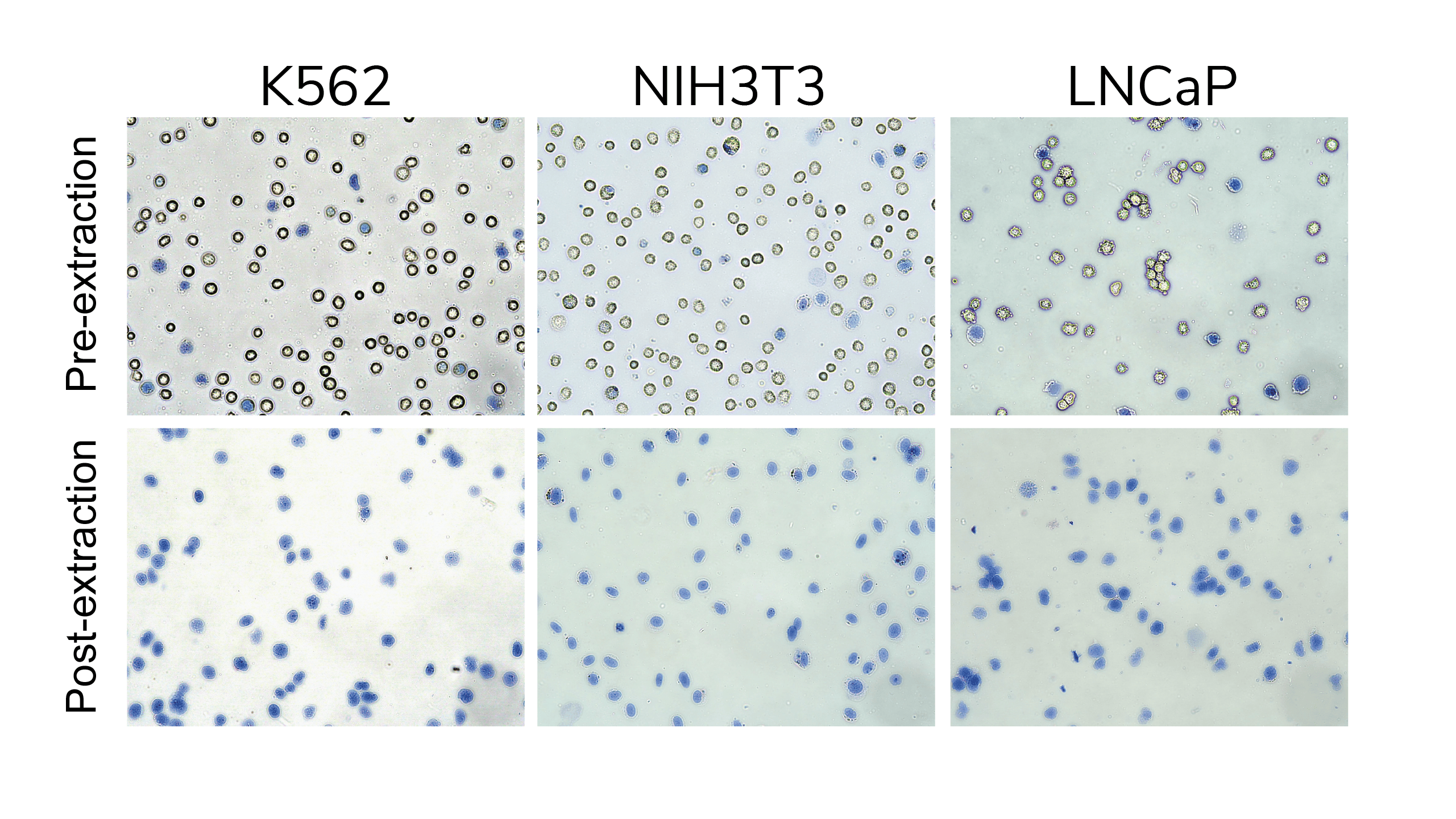
Figure 1. Nuclei successfully isolated with CUTANA Nuclei Extraction Protocol. Viable starting cells exclude Trypan Blue (top row), while isolated nuclei stain Trypan Blue positive (bottom row).
Nuclei Cryopreservation and Thawing Protocol
Aliquot nuclei resuspended in Nuclei Extraction Buffer. EpiCypher scientists typically aliquot for >8 reactions (800 µL + plus 20-30% excess) to account for sample loss at thaw steps.
Slowly freeze aliquots in an isopropanol-filled chiller in a -80°C freezer.
When ready to use samples, thaw nuclei by placing on ice for 5-10 minutes. Move quickly to avoid nuclear lysis and chromatin fragmentation. Note that only nuclei which have been in the -80°C freezer for at least 24 hours are recommended for thawing.
Thawed nuclei in Nuclei Extraction Buffer can be directly added to activated ConA beads in both CUT&RUN and CUT&Tag.