Important Notes
High quality sample prep is essential to CUT&RUN assay success and is the main variable we see when troubleshooting customer experiments. To ensure high quality sample prep, it is essential to examine cellular morphology, integrity, and viability at three steps:
Initial cell harvest: Cells have high viability and expected morphology.
Before ConA bead binding: Cells in CUT&RUN Wash Buffer have good integrity, minimal lysis, and/or cell loss.
After ConA bead binding: Majority of cells are permeabilized and bound to ConA beads.
Initial cell harvest
This refers to checking cell quality at the beginning of CUT&RUN, in the section Binding Cells to Activated Beads. Low starting cell viability, poor morphology, and cellular lysis increase assay background, so it is important to carefully examine each cell type.
Method: Count starting cells and determine viability using our Trypan Blue staining protocol. Examine cell integrity and morphology using a brightfield/phase microscope.
Expected results:
Cells should show expected morphology and high viability. For cultured cells, we recommend using cells that are ~70% confluent; do not use overgrown cells.
Note that viability may vary depending on your cell type (primary vs. cell line), if cells have been treated/stimulated, and other factors. For instance, K562 cells typically show >90% viability, while for other cell types or conditions, the optimal viability may be lower. Make the best decision based your sample type.
Next step in protocol: Harvest 500,000 viable cells per reaction, plus ~10% excess to account for sample loss.
Before ConA bead binding
This check is performed just prior to ConA bead binding, when cells are resuspended in Wash Buffer, and represents the final sample quality check before starting CUT&RUN. The purpose of this step is to confirm that washed cells (or nuclei) show normal morphology and remain intact, which is critical for CUT&RUN workflows. Data from lysed cells are not captured in CUT&RUN, and lysis may increase background noise.
Method: Determine total cell counts using our Trypan Blue staining protocol. Examine cell integrity and morphology using a brightfield/phase microscope.
Expected results:
Cells should show normal morphology/integrity, minimal cell lysis, and be unclumped.
Cells may show reduced viability in Wash Buffer compared to the initial cell harvest. Instead, focus on total cell counts, confirming ~500,000 cells per reaction.
Note: If using nuclei, nuclei should all be Trypan Blue positive at this step.
To troubleshoot cell loss: Increase spin time (keep at 600 x g). Leave ~50 μL liquid on cell pellet when removing supernatant to minimize cell loss, and gently pipette to resuspend.
Next step in protocol: Proceed to ConA bead binding.
After ConA bead binding
This step is to confirm cell permeabilization and ConA bead binding. In the section Binding Cells to Activated Beads, we instruct users to save 10 µL of supernatant following ConA bead binding (unbound fraction) and 10 µL of bead bound sample in Antibody Buffer (bead fraction). Note that Antibody Buffer contains Digitonin, which permeabilizes cells.
Method: Examine unbound fraction and bead fraction using our Trypan Blue staining protocol.
Expected results:
Little to no material is present in the unbound fraction (supernatant; Figure 1A).
In the bead fraction, >95% of cells (or nuclei) are Trypan Blue positive and surrounded by ConA beads (Figure 1B).
To troubleshoot: Ensure ConA beads were never frozen, cells/nuclei were not clumped, beads did not dry out, and all buffers were correctly prepared.
Next steps: Proceed to Antibody Binding section of the Manual (Section IV) or Protocol (Section III).
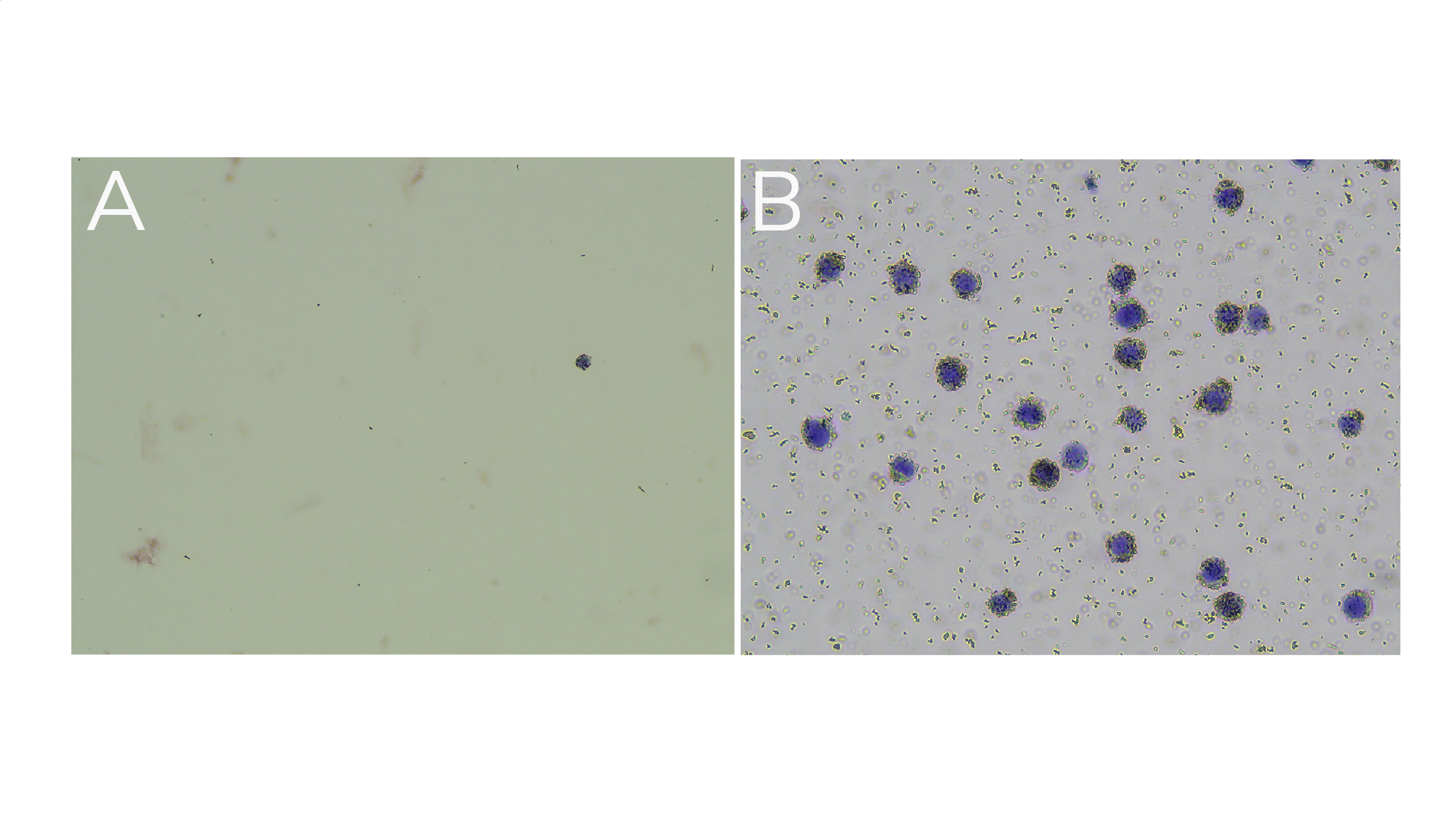
Figure 1. (A) Unbound fraction has minimal nuclei. (B) Representative sample slurry image showing nuclei (blue) successfully conjugated to activated ConA beads (brown specks).