This article also applies to meCUT&RUN and Multiomic CUT&RUN.
Important Notes
High-quality sample prep is essential for CUT&RUN experimental success. This guide is for fresh, native (i.e. unfixed, not frozen) suspension cell culture. For alternative sample types (i.e. adherent cultures, tissues, cross-linking, frozen samples), refer to other articles in this section.
Count starting cells and confirm cellular integrity, morphology, and viability. It is important that cells have good starting viability and expected morphology prior to being resuspended in CUT&RUN Wash Buffer. For K652 cells, we aim for >90% viability.
Harvest 500,000 live cells per reaction plus 10% excess. Spin at 600 x g for 3 min at room temperature (RT). Remove supernatant by pipetting, leaving a small amount of liquid on the pellet to avoid sample loss.
Resuspend cells in 100 µL per reaction RT Wash Buffer by gentle yet thorough pipetting. Spin at 600 x g for 3 min at RT. Pipette to remove supernatant.
Repeat Step 3 one time.
Resuspend cells in 105 µL per reaction RT Wash Buffer by gentle yet thorough pipetting.
Transfer 10 µL cells to a new 1.5 mL tube. Count and examine integrity of prepared cells by Trypan Blue staining. Note that some cell types show poor viability after resuspension in CUT&RUN Wash Buffer. In these instances, focus on total cell counts and cellular integrity. There should be minimal lysis, and you should have ~500,000 cells per reaction.
Add 100 µL cells to 10 µL ConA beads in 8-strip tubes. Gently vortex to mix and quick spin in a mini-centrifuge to collect slurry (beads should not settle).
Incubate bead-cell slurry for 10 min at RT to adsorb cells to beads.
Place tubes on a 8-strip tube magnetic rack and allow slurry to clear.
If bead binding was successful, the supernatant should not contain cells. To confirm, transfer 10 µL supernatant to a new 1.5 mL tube and perform Trypan Blue staining (see Figure 1A for example data).
Discard remaining supernatant and move quickly to the next step. Do not allow beads to dry out.
Remove tubes from the magnet. Immediately add 50 µL cold Antibody Buffer to each reaction and pipette to resuspend.
Transfer 10 µL of bead-cell mixture (reaction slurry) to a new 1.5 mL tube.
Examine reaction slurry using Trypan Blue staining to confirm ConA bead binding. The bead-cell slurry should contain cells bound to beads (Figure 1B).
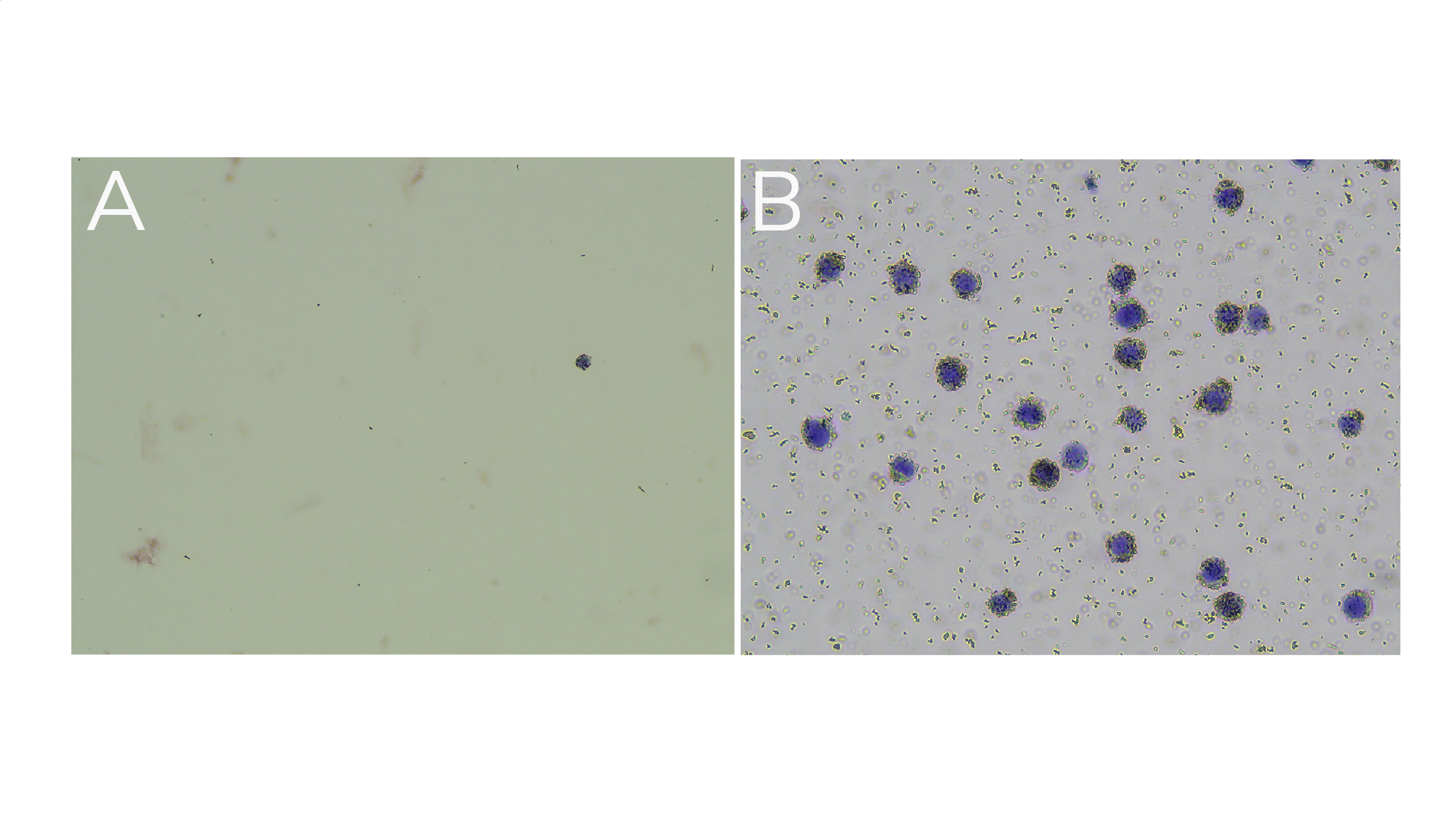
Figure 1. (A) Supernatant shows little to no material leftover after ConA bead conjugation. (B) Representative reaction slurry image showing nuclei (blue) successfully conjugated to activated ConA beads (brown specs). Note: ConA bead-bound cells will also appear Trypan Blue positive due to the presence of Digitonin in the Antibody Buffer.